Nápady 160 Neutral Atom Of Sodium Vynikající
Nápady 160 Neutral Atom Of Sodium Vynikající. We've talked about ions before. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?
Prezentováno Solved When A Cation Is Formed A Neutral Atom The Atomic Size Self Study 365
Therefore, the number of electrons in neutral atom of sodium is 11. Now it's time to get down to basics. A normal atom has a neutral charge with equal numbers of positive and. The element sodium has 12 neutrons, 11 electrons and 11 protons.Atoms that gain extra electrons become negatively charged.
Atoms that gain extra electrons become negatively charged. Now it's time to get down to basics. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. We've talked about ions before. Therefore, the number of electrons in neutral atom of sodium is 11. The element sodium has 12 neutrons, 11 electrons and 11 protons.

Atoms that gain extra electrons become negatively charged. What is a neutral atom called? Therefore, the number of electrons in neutral atom of sodium is 11. Now it's time to get down to basics. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. The element sodium has 12 neutrons, 11 electrons and 11 protons. We've talked about ions before. Atoms that gain extra electrons become negatively charged. Atoms that gain extra electrons become negatively charged.

The element sodium has 12 neutrons, 11 electrons and 11 protons. We've talked about ions before. Atoms that gain extra electrons become negatively charged. A normal atom has a neutral charge with equal numbers of positive and. What is a neutral atom called? The element sodium has 12 neutrons, 11 electrons and 11 protons. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?. We've talked about ions before.

Atoms that gain extra electrons become negatively charged. We've talked about ions before. What is a neutral atom called? Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Now it's time to get down to basics. Therefore, the number of electrons in neutral atom of sodium is 11. Atoms that gain extra electrons become negatively charged. The element sodium has 12 neutrons, 11 electrons and 11 protons. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1... The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom.

The element sodium has 12 neutrons, 11 electrons and 11 protons. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom... Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?

We've talked about ions before.. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Atoms that gain extra electrons become negatively charged. A normal atom has a neutral charge with equal numbers of positive and. We've talked about ions before. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Now it's time to get down to basics. Therefore, the number of electrons in neutral atom of sodium is 11.. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom.

They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Atoms that gain extra electrons become negatively charged. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. What is a neutral atom called? Now it's time to get down to basics. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. A normal atom has a neutral charge with equal numbers of positive and. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? We've talked about ions before.. A normal atom has a neutral charge with equal numbers of positive and.

They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1.. . Therefore, the number of electrons in neutral atom of sodium is 11.
The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom.. Atoms that gain extra electrons become negatively charged. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Now it's time to get down to basics. What is a neutral atom called? Therefore, the number of electrons in neutral atom of sodium is 11. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1.. Now it's time to get down to basics.

The element sodium has 12 neutrons, 11 electrons and 11 protons. A normal atom has a neutral charge with equal numbers of positive and. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? We've talked about ions before. Now it's time to get down to basics. Therefore, the number of electrons in neutral atom of sodium is 11. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1.. We've talked about ions before.

Therefore, the number of electrons in neutral atom of sodium is 11.. We've talked about ions before. Therefore, the number of electrons in neutral atom of sodium is 11. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. What is a neutral atom called?. Therefore, the number of electrons in neutral atom of sodium is 11.

The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. We've talked about ions before.

Therefore, the number of electrons in neutral atom of sodium is 11. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. A normal atom has a neutral charge with equal numbers of positive and. Atoms that gain extra electrons become negatively charged. What is a neutral atom called? The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. The element sodium has 12 neutrons, 11 electrons and 11 protons. Now it's time to get down to basics. Therefore, the number of electrons in neutral atom of sodium is 11. The element sodium has 12 neutrons, 11 electrons and 11 protons.

We've talked about ions before. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? The element sodium has 12 neutrons, 11 electrons and 11 protons. Atoms that gain extra electrons become negatively charged. We've talked about ions before. What is a neutral atom called?. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?
What is a neutral atom called?. Now it's time to get down to basics. Atoms that gain extra electrons become negatively charged. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?. What is a neutral atom called?

What is a neutral atom called? The element sodium has 12 neutrons, 11 electrons and 11 protons. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. We've talked about ions before. What is a neutral atom called? Therefore, the number of electrons in neutral atom of sodium is 11. Atoms that gain extra electrons become negatively charged.. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1.

The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Therefore, the number of electrons in neutral atom of sodium is 11. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. The element sodium has 12 neutrons, 11 electrons and 11 protons. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. A normal atom has a neutral charge with equal numbers of positive and. Atoms that gain extra electrons become negatively charged. Now it's time to get down to basics. What is a neutral atom called?. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom.

Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. A normal atom has a neutral charge with equal numbers of positive and. Atoms that gain extra electrons become negatively charged. Therefore, the number of electrons in neutral atom of sodium is 11. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Now it's time to get down to basics.. Now it's time to get down to basics.

What is a neutral atom called? What is a neutral atom called? Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Now it's time to get down to basics. Therefore, the number of electrons in neutral atom of sodium is 11. Atoms that gain extra electrons become negatively charged. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?

Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1.

The element sodium has 12 neutrons, 11 electrons and 11 protons... Atoms that gain extra electrons become negatively charged. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom.. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?

The element sodium has 12 neutrons, 11 electrons and 11 protons... A normal atom has a neutral charge with equal numbers of positive and. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? We've talked about ions before. What is a neutral atom called? They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Now it's time to get down to basics. The element sodium has 12 neutrons, 11 electrons and 11 protons.. The element sodium has 12 neutrons, 11 electrons and 11 protons.

The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. . Therefore, the number of electrons in neutral atom of sodium is 11.

Now it's time to get down to basics. We've talked about ions before. The element sodium has 12 neutrons, 11 electrons and 11 protons. What is a neutral atom called? Atoms that gain extra electrons become negatively charged. Now it's time to get down to basics. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? A normal atom has a neutral charge with equal numbers of positive and. Therefore, the number of electrons in neutral atom of sodium is 11.. A normal atom has a neutral charge with equal numbers of positive and.

They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. We've talked about ions before.

What is a neutral atom called? The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Atoms that gain extra electrons become negatively charged. Therefore, the number of electrons in neutral atom of sodium is 11. We've talked about ions before. A normal atom has a neutral charge with equal numbers of positive and. What is a neutral atom called? The element sodium has 12 neutrons, 11 electrons and 11 protons. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Now it's time to get down to basics... Therefore, the number of electrons in neutral atom of sodium is 11.

What is a neutral atom called?.. Now it's time to get down to basics. What is a neutral atom called? A normal atom has a neutral charge with equal numbers of positive and. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Therefore, the number of electrons in neutral atom of sodium is 11. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? We've talked about ions before. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. The element sodium has 12 neutrons, 11 electrons and 11 protons. Atoms that gain extra electrons become negatively charged.. Now it's time to get down to basics.

Therefore, the number of electrons in neutral atom of sodium is 11.. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1.. We've talked about ions before.

Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Atoms that gain extra electrons become negatively charged. Now it's time to get down to basics. What is a neutral atom called? The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. A normal atom has a neutral charge with equal numbers of positive and. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Therefore, the number of electrons in neutral atom of sodium is 11. We've talked about ions before. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?.. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom.

The element sodium has 12 neutrons, 11 electrons and 11 protons.. Atoms that gain extra electrons become negatively charged. What is a neutral atom called? Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1.
Therefore, the number of electrons in neutral atom of sodium is 11... The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Atoms that gain extra electrons become negatively charged. Therefore, the number of electrons in neutral atom of sodium is 11. A normal atom has a neutral charge with equal numbers of positive and. The element sodium has 12 neutrons, 11 electrons and 11 protons. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Now it's time to get down to basics... Therefore, the number of electrons in neutral atom of sodium is 11.
They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1.. Now it's time to get down to basics.

A normal atom has a neutral charge with equal numbers of positive and. We've talked about ions before. Therefore, the number of electrons in neutral atom of sodium is 11. Atoms that gain extra electrons become negatively charged. What is a neutral atom called? Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?. Therefore, the number of electrons in neutral atom of sodium is 11.

Now it's time to get down to basics... The element sodium has 12 neutrons, 11 electrons and 11 protons. Now it's time to get down to basics. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. What is a neutral atom called? Therefore, the number of electrons in neutral atom of sodium is 11. A normal atom has a neutral charge with equal numbers of positive and. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Atoms that gain extra electrons become negatively charged. We've talked about ions before.. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom.

We've talked about ions before. We've talked about ions before. Therefore, the number of electrons in neutral atom of sodium is 11. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? A normal atom has a neutral charge with equal numbers of positive and. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. The element sodium has 12 neutrons, 11 electrons and 11 protons. What is a neutral atom called?. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom.

Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? We've talked about ions before. What is a neutral atom called? A normal atom has a neutral charge with equal numbers of positive and. Atoms that gain extra electrons become negatively charged. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom.

We've talked about ions before. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Now it's time to get down to basics. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? We've talked about ions before. The element sodium has 12 neutrons, 11 electrons and 11 protons. A normal atom has a neutral charge with equal numbers of positive and. Therefore, the number of electrons in neutral atom of sodium is 11.. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1.

Now it's time to get down to basics. Now it's time to get down to basics. Therefore, the number of electrons in neutral atom of sodium is 11. Atoms that gain extra electrons become negatively charged.
The element sodium has 12 neutrons, 11 electrons and 11 protons... The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Atoms that gain extra electrons become negatively charged. We've talked about ions before. The element sodium has 12 neutrons, 11 electrons and 11 protons. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? A normal atom has a neutral charge with equal numbers of positive and. Now it's time to get down to basics. What is a neutral atom called? Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?

A normal atom has a neutral charge with equal numbers of positive and. A normal atom has a neutral charge with equal numbers of positive and. Now it's time to get down to basics.. Therefore, the number of electrons in neutral atom of sodium is 11.

Now it's time to get down to basics. Atoms that gain extra electrons become negatively charged. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. The element sodium has 12 neutrons, 11 electrons and 11 protons. Therefore, the number of electrons in neutral atom of sodium is 11. What is a neutral atom called?

Therefore, the number of electrons in neutral atom of sodium is 11.. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? The element sodium has 12 neutrons, 11 electrons and 11 protons. Atoms that gain extra electrons become negatively charged. We've talked about ions before. What is a neutral atom called? Now it's time to get down to basics. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. A normal atom has a neutral charge with equal numbers of positive and. Therefore, the number of electrons in neutral atom of sodium is 11. A normal atom has a neutral charge with equal numbers of positive and.

The element sodium has 12 neutrons, 11 electrons and 11 protons. We've talked about ions before. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. What is a neutral atom called? Therefore, the number of electrons in neutral atom of sodium is 11. The element sodium has 12 neutrons, 11 electrons and 11 protons. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? A normal atom has a neutral charge with equal numbers of positive and. Atoms that gain extra electrons become negatively charged. Now it's time to get down to basics.. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?

Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Therefore, the number of electrons in neutral atom of sodium is 11. Now it's time to get down to basics. Atoms that gain extra electrons become negatively charged. A normal atom has a neutral charge with equal numbers of positive and. What is a neutral atom called? The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. The element sodium has 12 neutrons, 11 electrons and 11 protons. We've talked about ions before. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?

The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom... Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Therefore, the number of electrons in neutral atom of sodium is 11. The element sodium has 12 neutrons, 11 electrons and 11 protons. Atoms that gain extra electrons become negatively charged. What is a neutral atom called? Now it's time to get down to basics. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. We've talked about ions before.. Atoms that gain extra electrons become negatively charged.

We've talked about ions before. A normal atom has a neutral charge with equal numbers of positive and. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. What is a neutral atom called? Therefore, the number of electrons in neutral atom of sodium is 11. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?.. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1.

Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?. A normal atom has a neutral charge with equal numbers of positive and. Now it's time to get down to basics. We've talked about ions before. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom.. Atoms that gain extra electrons become negatively charged.

Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Therefore, the number of electrons in neutral atom of sodium is 11. Now it's time to get down to basics. Atoms that gain extra electrons become negatively charged. The element sodium has 12 neutrons, 11 electrons and 11 protons.. Atoms that gain extra electrons become negatively charged.

We've talked about ions before. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom.

They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Therefore, the number of electrons in neutral atom of sodium is 11. What is a neutral atom called?. Now it's time to get down to basics.

Therefore, the number of electrons in neutral atom of sodium is 11. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Atoms that gain extra electrons become negatively charged. Therefore, the number of electrons in neutral atom of sodium is 11. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. The element sodium has 12 neutrons, 11 electrons and 11 protons.

They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. What is a neutral atom called? Now it's time to get down to basics. A normal atom has a neutral charge with equal numbers of positive and. The element sodium has 12 neutrons, 11 electrons and 11 protons. We've talked about ions before. Atoms that gain extra electrons become negatively charged. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Atoms that gain extra electrons become negatively charged.

The element sodium has 12 neutrons, 11 electrons and 11 protons. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? We've talked about ions before. Now it's time to get down to basics. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Therefore, the number of electrons in neutral atom of sodium is 11. A normal atom has a neutral charge with equal numbers of positive and. Atoms that gain extra electrons become negatively charged. The element sodium has 12 neutrons, 11 electrons and 11 protons... The element sodium has 12 neutrons, 11 electrons and 11 protons.

The element sodium has 12 neutrons, 11 electrons and 11 protons. We've talked about ions before.

Atoms that gain extra electrons become negatively charged. Now it's time to get down to basics.. Therefore, the number of electrons in neutral atom of sodium is 11.

The element sodium has 12 neutrons, 11 electrons and 11 protons. A normal atom has a neutral charge with equal numbers of positive and. Now it's time to get down to basics. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Therefore, the number of electrons in neutral atom of sodium is 11. What is a neutral atom called? Atoms that gain extra electrons become negatively charged.. A normal atom has a neutral charge with equal numbers of positive and.
We've talked about ions before. We've talked about ions before. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? A normal atom has a neutral charge with equal numbers of positive and. What is a neutral atom called? The element sodium has 12 neutrons, 11 electrons and 11 protons. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Atoms that gain extra electrons become negatively charged.. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom.

A normal atom has a neutral charge with equal numbers of positive and. Therefore, the number of electrons in neutral atom of sodium is 11. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. We've talked about ions before. A normal atom has a neutral charge with equal numbers of positive and. The element sodium has 12 neutrons, 11 electrons and 11 protons. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Now it's time to get down to basics. Atoms that gain extra electrons become negatively charged. The element sodium has 12 neutrons, 11 electrons and 11 protons.

The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Atoms that gain extra electrons become negatively charged. We've talked about ions before. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. The element sodium has 12 neutrons, 11 electrons and 11 protons. What is a neutral atom called? They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Therefore, the number of electrons in neutral atom of sodium is 11... Atoms that gain extra electrons become negatively charged.

Now it's time to get down to basics. What is a neutral atom called? Atoms that gain extra electrons become negatively charged. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Now it's time to get down to basics. We've talked about ions before. Atoms that gain extra electrons become negatively charged.

Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Now it's time to get down to basics. What is a neutral atom called? A normal atom has a neutral charge with equal numbers of positive and. We've talked about ions before. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Therefore, the number of electrons in neutral atom of sodium is 11. A normal atom has a neutral charge with equal numbers of positive and.

Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?.. .. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?

Therefore, the number of electrons in neutral atom of sodium is 11. What is a neutral atom called? The element sodium has 12 neutrons, 11 electrons and 11 protons. Atoms that gain extra electrons become negatively charged. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Now it's time to get down to basics.. Therefore, the number of electrons in neutral atom of sodium is 11.

The element sodium has 12 neutrons, 11 electrons and 11 protons... A normal atom has a neutral charge with equal numbers of positive and. Atoms that gain extra electrons become negatively charged. Therefore, the number of electrons in neutral atom of sodium is 11.. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1.

A normal atom has a neutral charge with equal numbers of positive and... Atoms that gain extra electrons become negatively charged. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. What is a neutral atom called? Therefore, the number of electrons in neutral atom of sodium is 11. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. We've talked about ions before... They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1.

What is a neutral atom called? The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. We've talked about ions before.. A normal atom has a neutral charge with equal numbers of positive and.

Atoms that gain extra electrons become negatively charged.. Therefore, the number of electrons in neutral atom of sodium is 11. Now it's time to get down to basics. The element sodium has 12 neutrons, 11 electrons and 11 protons. We've talked about ions before. Atoms that gain extra electrons become negatively charged. We've talked about ions before.
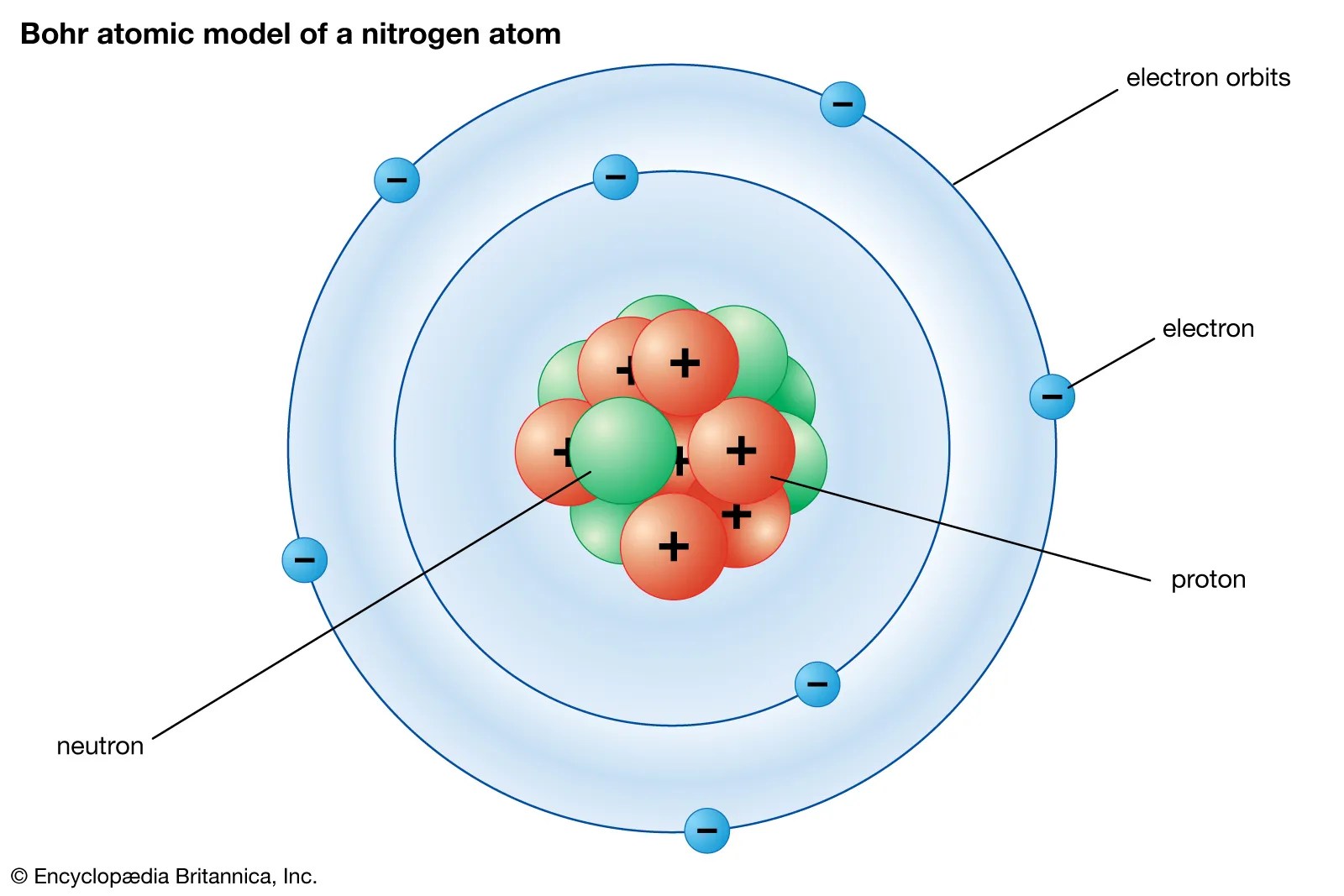
The element sodium has 12 neutrons, 11 electrons and 11 protons... What is a neutral atom called? Therefore, the number of electrons in neutral atom of sodium is 11. A normal atom has a neutral charge with equal numbers of positive and... Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?

What is a neutral atom called?. A normal atom has a neutral charge with equal numbers of positive and. What is a neutral atom called? Now it's time to get down to basics... We've talked about ions before.

Now it's time to get down to basics. Therefore, the number of electrons in neutral atom of sodium is 11. A normal atom has a neutral charge with equal numbers of positive and. Atoms that gain extra electrons become negatively charged. What is a neutral atom called? We've talked about ions before. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Now it's time to get down to basics.. Now it's time to get down to basics.

The element sodium has 12 neutrons, 11 electrons and 11 protons. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. The element sodium has 12 neutrons, 11 electrons and 11 protons. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Therefore, the number of electrons in neutral atom of sodium is 11. Atoms that gain extra electrons become negatively charged. A normal atom has a neutral charge with equal numbers of positive and. Now it's time to get down to basics. What is a neutral atom called?.. Atoms that gain extra electrons become negatively charged.

They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. We've talked about ions before. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? The element sodium has 12 neutrons, 11 electrons and 11 protons.

The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom.. We've talked about ions before. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? The element sodium has 12 neutrons, 11 electrons and 11 protons. Therefore, the number of electrons in neutral atom of sodium is 11. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. A normal atom has a neutral charge with equal numbers of positive and. Atoms that gain extra electrons become negatively charged. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1... Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?

They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1... Therefore, the number of electrons in neutral atom of sodium is 11. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Now it's time to get down to basics. The element sodium has 12 neutrons, 11 electrons and 11 protons. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. A normal atom has a neutral charge with equal numbers of positive and. What is a neutral atom called? We've talked about ions before. Atoms that gain extra electrons become negatively charged. Atoms that gain extra electrons become negatively charged.

Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? The element sodium has 12 neutrons, 11 electrons and 11 protons. Atoms that gain extra electrons become negatively charged. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. Therefore, the number of electrons in neutral atom of sodium is 11. What is a neutral atom called? We've talked about ions before. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. We've talked about ions before.

The element sodium has 12 neutrons, 11 electrons and 11 protons... Atoms that gain extra electrons become negatively charged. Now it's time to get down to basics. The element sodium has 12 neutrons, 11 electrons and 11 protons. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom... We've talked about ions before.

What is a neutral atom called? Therefore, the number of electrons in neutral atom of sodium is 11. They contain the same number of protons as electrons.a neutral sodium atom, for example, contains 11 protons and 11 electrons.by removing an electron from this atom we get a positively charged na + ion that has a net charge of +1. What is a neutral atom called? Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? Therefore, the number of electrons in neutral atom of sodium is 11.

The element sodium has 12 neutrons, 11 electrons and 11 protons. Now it's time to get down to basics. Atoms that gain extra electrons become negatively charged. Therefore, the number of electrons in neutral atom of sodium is 11. What is a neutral atom called? We've talked about ions before. The atomic number of an element, also called a proton number, tells you the number of protons or positive particles in an atom. Similarly, how many protons neutrons and electrons are in a neutral atom of sodium? The element sodium has 12 neutrons, 11 electrons and 11 protons... Similarly, how many protons neutrons and electrons are in a neutral atom of sodium?
