Seznamy Atom Economy Equation
Seznamy Atom Economy Equation. Table 4 experimental atom economy of equation 1: Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The greater the value of the %atom economy, the less the amount of waste product produced.
Prezentováno Calculating Atom Economy Aqa Gcse Chemistry Revision Notes
Substitution reactions don't have 100% atom economy as there is more than one product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Total mass of all reactants = mass of desired product + mass of waste products. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. It is important for sustainable development and for economic reasons to use.It is important for sustainable development and for economic reasons to use.
Of a reaction is a measure of the amount of starting materials that end up as useful products. Total mass of all reactants = mass of desired product + mass of waste products. Of a reaction is a measure of how many reactant atoms form a desired product. Equation (i) is identical to equation (ii) because by the law of mass conservation: May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

Table 4 experimental atom economy of equation 1: Total mass of all reactants = mass of desired product + mass of waste products... Total mass of all reactants = mass of desired product + mass of waste products.

Equation (i) is identical to equation (ii) because by the law of mass conservation: It is important for sustainable development and for economic reasons to use. Total mass of all reactants = mass of desired product + mass of waste products. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.

Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): It is important for sustainable development and for economic reasons to use. Based on actual quantities of reagents used. Total mass of all reactants = mass of desired product + mass of waste products. Of a reaction is a measure of the amount of starting materials that end up as useful products. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Table 4 experimental atom economy of equation 1: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The greater the value of the %atom economy, the less the amount of waste product produced. Equation (i) is identical to equation (ii) because by the law of mass conservation:

The greater the value of the %atom economy, the less the amount of waste product produced.. . May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.

Of a reaction is a measure of the amount of starting materials that end up as useful products... May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. The greater the value of the %atom economy, the less the amount of waste product produced. Based on actual quantities of reagents used. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Total mass of all reactants = mass of desired product + mass of waste products. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The percentage atom economy of a reaction is calculated using this equation: Of a reaction is a measure of the amount of starting materials that end up as useful products. Of a reaction is a measure of the amount of starting materials that end up as useful products.

The percentage atom economy of a reaction is calculated using this equation: The greater the value of the %atom economy, the less the amount of waste product produced. The percentage atom economy of a reaction is calculated using this equation: Table 4 experimental atom economy of equation 1: Substitution reactions don't have 100% atom economy as there is more than one product. Equation (i) is identical to equation (ii) because by the law of mass conservation: Of a reaction is a measure of how many reactant atoms form a desired product. Of a reaction is a measure of the amount of starting materials that end up as useful products. Total mass of all reactants = mass of desired product + mass of waste products. It is important for sustainable development and for economic reasons to use. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Of a reaction is a measure of how many reactant atoms form a desired product.

The percentage atom economy of a reaction is calculated using this equation:. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Equation (i) is identical to equation (ii) because by the law of mass conservation: May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. It is important for sustainable development and for economic reasons to use. Equation (i) is identical to equation (ii) because by the law of mass conservation:

Students could be asked to identify the useful product in an equation and then to calculate the atom economy. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Based on actual quantities of reagents used. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Equation (i) is identical to equation (ii) because by the law of mass conservation: It is important for sustainable development and for economic reasons to use. Total mass of all reactants = mass of desired product + mass of waste products. The greater the value of the %atom economy, the less the amount of waste product produced. Of a reaction is a measure of the amount of starting materials that end up as useful products. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Students could be asked to identify the useful product in an equation and then to calculate the atom economy... Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):

Equation (i) is identical to equation (ii) because by the law of mass conservation:. Equation (i) is identical to equation (ii) because by the law of mass conservation: It is important for sustainable development and for economic reasons to use. Of a reaction is a measure of the amount of starting materials that end up as useful products. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The greater the value of the %atom economy, the less the amount of waste product produced. Based on actual quantities of reagents used. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Table 4 experimental atom economy of equation 1: It is important for sustainable development and for economic reasons to use.

Equation (i) is identical to equation (ii) because by the law of mass conservation:. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Of a reaction is a measure of how many reactant atoms form a desired product. Of a reaction is a measure of the amount of starting materials that end up as useful products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Based on actual quantities of reagents used. Total mass of all reactants = mass of desired product + mass of waste products.. Equation (i) is identical to equation (ii) because by the law of mass conservation:
Equation (i) is identical to equation (ii) because by the law of mass conservation:. Based on actual quantities of reagents used. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Of a reaction is a measure of how many reactant atoms form a desired product. Table 4 experimental atom economy of equation 1: May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. The greater the value of the %atom economy, the less the amount of waste product produced. Substitution reactions don't have 100% atom economy as there is more than one product. It is important for sustainable development and for economic reasons to use.. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):
Based on actual quantities of reagents used. Equation (i) is identical to equation (ii) because by the law of mass conservation: The percentage atom economy of a reaction is calculated using this equation: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Total mass of all reactants = mass of desired product + mass of waste products. Based on actual quantities of reagents used. Substitution reactions don't have 100% atom economy as there is more than one product. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Of a reaction is a measure of the amount of starting materials that end up as useful products. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Based on actual quantities of reagents used. It is important for sustainable development and for economic reasons to use. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):. Of a reaction is a measure of how many reactant atoms form a desired product.

May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. The percentage atom economy of a reaction is calculated using this equation: Students could be asked to identify the useful product in an equation and then to calculate the atom economy... The greater the value of the %atom economy, the less the amount of waste product produced.

Of a reaction is a measure of the amount of starting materials that end up as useful products... Of a reaction is a measure of the amount of starting materials that end up as useful products. Substitution reactions don't have 100% atom economy as there is more than one product. Equation (i) is identical to equation (ii) because by the law of mass conservation: Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. Substitution reactions don't have 100% atom economy as there is more than one product.

Total mass of all reactants = mass of desired product + mass of waste products. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Based on actual quantities of reagents used. Equation (i) is identical to equation (ii) because by the law of mass conservation: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Table 4 experimental atom economy of equation 1: Of a reaction is a measure of the amount of starting materials that end up as useful products. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):.. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Of a reaction is a measure of how many reactant atoms form a desired product. Based on actual quantities of reagents used.. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

It is important for sustainable development and for economic reasons to use. It is important for sustainable development and for economic reasons to use. Of a reaction is a measure of how many reactant atoms form a desired product. Based on actual quantities of reagents used. The greater the value of the %atom economy, the less the amount of waste product produced. Of a reaction is a measure of the amount of starting materials that end up as useful products. Table 4 experimental atom economy of equation 1: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

Total mass of all reactants = mass of desired product + mass of waste products. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. It is important for sustainable development and for economic reasons to use. Equation (i) is identical to equation (ii) because by the law of mass conservation: Total mass of all reactants = mass of desired product + mass of waste products. Table 4 experimental atom economy of equation 1:

May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.. Equation (i) is identical to equation (ii) because by the law of mass conservation: Substitution reactions don't have 100% atom economy as there is more than one product. It is important for sustainable development and for economic reasons to use. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Of a reaction is a measure of how many reactant atoms form a desired product. The greater the value of the %atom economy, the less the amount of waste product produced.
The greater the value of the %atom economy, the less the amount of waste product produced. It is important for sustainable development and for economic reasons to use. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):

It is important for sustainable development and for economic reasons to use. . Total mass of all reactants = mass of desired product + mass of waste products.

Table 4 experimental atom economy of equation 1: Equation (i) is identical to equation (ii) because by the law of mass conservation: The greater the value of the %atom economy, the less the amount of waste product produced. Based on actual quantities of reagents used. Substitution reactions don't have 100% atom economy as there is more than one product. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Of a reaction is a measure of how many reactant atoms form a desired product. Table 4 experimental atom economy of equation 1: It is important for sustainable development and for economic reasons to use. Total mass of all reactants = mass of desired product + mass of waste products. Based on actual quantities of reagents used.

Equation (i) is identical to equation (ii) because by the law of mass conservation:. Of a reaction is a measure of how many reactant atoms form a desired product. It is important for sustainable development and for economic reasons to use. Equation (i) is identical to equation (ii) because by the law of mass conservation: Total mass of all reactants = mass of desired product + mass of waste products. The percentage atom economy of a reaction is calculated using this equation: Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The greater the value of the %atom economy, the less the amount of waste product produced. Of a reaction is a measure of the amount of starting materials that end up as useful products. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.. Equation (i) is identical to equation (ii) because by the law of mass conservation:

Based on actual quantities of reagents used. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Equation (i) is identical to equation (ii) because by the law of mass conservation: Of a reaction is a measure of the amount of starting materials that end up as useful products. The greater the value of the %atom economy, the less the amount of waste product produced. The percentage atom economy of a reaction is calculated using this equation: May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.

Of a reaction is a measure of how many reactant atoms form a desired product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Of a reaction is a measure of the amount of starting materials that end up as useful products. The percentage atom economy of a reaction is calculated using this equation: The greater the value of the %atom economy, the less the amount of waste product produced. It is important for sustainable development and for economic reasons to use.

Based on actual quantities of reagents used. Table 4 experimental atom economy of equation 1:

May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.. . Of a reaction is a measure of how many reactant atoms form a desired product.

Of a reaction is a measure of the amount of starting materials that end up as useful products.. .. Of a reaction is a measure of the amount of starting materials that end up as useful products.

Equation (i) is identical to equation (ii) because by the law of mass conservation: Total mass of all reactants = mass of desired product + mass of waste products. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Based on actual quantities of reagents used. Of a reaction is a measure of the amount of starting materials that end up as useful products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Substitution reactions don't have 100% atom economy as there is more than one product. The greater the value of the %atom economy, the less the amount of waste product produced. Table 4 experimental atom economy of equation 1: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Of a reaction is a measure of how many reactant atoms form a desired product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.
Based on actual quantities of reagents used. The greater the value of the %atom economy, the less the amount of waste product produced. Of a reaction is a measure of the amount of starting materials that end up as useful products. Based on actual quantities of reagents used. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Table 4 experimental atom economy of equation 1: May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Equation (i) is identical to equation (ii) because by the law of mass conservation: Total mass of all reactants = mass of desired product + mass of waste products. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Based on actual quantities of reagents used.

Table 4 experimental atom economy of equation 1: Substitution reactions don't have 100% atom economy as there is more than one product. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Based on actual quantities of reagents used. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Equation (i) is identical to equation (ii) because by the law of mass conservation: Of a reaction is a measure of how many reactant atoms form a desired product. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. It is important for sustainable development and for economic reasons to use. Total mass of all reactants = mass of desired product + mass of waste products. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Table 4 experimental atom economy of equation 1: Substitution reactions don't have 100% atom economy as there is more than one product.. Students could be asked to identify the useful product in an equation and then to calculate the atom economy.

May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Table 4 experimental atom economy of equation 1: Of a reaction is a measure of the amount of starting materials that end up as useful products. Total mass of all reactants = mass of desired product + mass of waste products. Equation (i) is identical to equation (ii) because by the law of mass conservation: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Substitution reactions don't have 100% atom economy as there is more than one product... Substitution reactions don't have 100% atom economy as there is more than one product.

Of a reaction is a measure of how many reactant atoms form a desired product. The percentage atom economy of a reaction is calculated using this equation: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. Equation (i) is identical to equation (ii) because by the law of mass conservation: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Substitution reactions don't have 100% atom economy as there is more than one product. Of a reaction is a measure of the amount of starting materials that end up as useful products. Total mass of all reactants = mass of desired product + mass of waste products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

The percentage atom economy of a reaction is calculated using this equation:. Of a reaction is a measure of the amount of starting materials that end up as useful products. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):

Of a reaction is a measure of how many reactant atoms form a desired product... Of a reaction is a measure of how many reactant atoms form a desired product. Substitution reactions don't have 100% atom economy as there is more than one product. Total mass of all reactants = mass of desired product + mass of waste products. It is important for sustainable development and for economic reasons to use. Of a reaction is a measure of the amount of starting materials that end up as useful products. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Of a reaction is a measure of how many reactant atoms form a desired product.

The greater the value of the %atom economy, the less the amount of waste product produced. Of a reaction is a measure of the amount of starting materials that end up as useful products. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100.. Substitution reactions don't have 100% atom economy as there is more than one product.

Based on actual quantities of reagents used. The percentage atom economy of a reaction is calculated using this equation: Total mass of all reactants = mass of desired product + mass of waste products. Substitution reactions don't have 100% atom economy as there is more than one product. Of a reaction is a measure of how many reactant atoms form a desired product. The greater the value of the %atom economy, the less the amount of waste product produced. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Of a reaction is a measure of the amount of starting materials that end up as useful products... Based on actual quantities of reagents used.

It is important for sustainable development and for economic reasons to use... Of a reaction is a measure of the amount of starting materials that end up as useful products. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Based on actual quantities of reagents used. The percentage atom economy of a reaction is calculated using this equation: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Of a reaction is a measure of how many reactant atoms form a desired product. Table 4 experimental atom economy of equation 1: Substitution reactions don't have 100% atom economy as there is more than one product. It is important for sustainable development and for economic reasons to use. Based on actual quantities of reagents used.

May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Of a reaction is a measure of the amount of starting materials that end up as useful products. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The percentage atom economy of a reaction is calculated using this equation: Table 4 experimental atom economy of equation 1: Equation (i) is identical to equation (ii) because by the law of mass conservation: It is important for sustainable development and for economic reasons to use.. Based on actual quantities of reagents used.

% atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Of a reaction is a measure of how many reactant atoms form a desired product. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Table 4 experimental atom economy of equation 1: The greater the value of the %atom economy, the less the amount of waste product produced. Substitution reactions don't have 100% atom economy as there is more than one product. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Total mass of all reactants = mass of desired product + mass of waste products.. The greater the value of the %atom economy, the less the amount of waste product produced.

It is important for sustainable development and for economic reasons to use. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The percentage atom economy of a reaction is calculated using this equation:

The greater the value of the %atom economy, the less the amount of waste product produced.. Total mass of all reactants = mass of desired product + mass of waste products. Substitution reactions don't have 100% atom economy as there is more than one product. It is important for sustainable development and for economic reasons to use. The greater the value of the %atom economy, the less the amount of waste product produced. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): Equation (i) is identical to equation (ii) because by the law of mass conservation: Based on actual quantities of reagents used. Of a reaction is a measure of the amount of starting materials that end up as useful products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. The percentage atom economy of a reaction is calculated using this equation: Equation (i) is identical to equation (ii) because by the law of mass conservation:

Based on actual quantities of reagents used... Equation (i) is identical to equation (ii) because by the law of mass conservation: Substitution reactions don't have 100% atom economy as there is more than one product. Total mass of all reactants = mass of desired product + mass of waste products... Of a reaction is a measure of how many reactant atoms form a desired product.

May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100... Table 4 experimental atom economy of equation 1: Students could be asked to identify the useful product in an equation and then to calculate the atom economy. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Of a reaction is a measure of how many reactant atoms form a desired product. Equation (i) is identical to equation (ii) because by the law of mass conservation: Based on actual quantities of reagents used. Substitution reactions don't have 100% atom economy as there is more than one product. It is important for sustainable development and for economic reasons to use. Of a reaction is a measure of the amount of starting materials that end up as useful products. Total mass of all reactants = mass of desired product + mass of waste products.

The greater the value of the %atom economy, the less the amount of waste product produced.. Of a reaction is a measure of the amount of starting materials that end up as useful products. Total mass of all reactants = mass of desired product + mass of waste products. The greater the value of the %atom economy, the less the amount of waste product produced. Substitution reactions don't have 100% atom economy as there is more than one product.
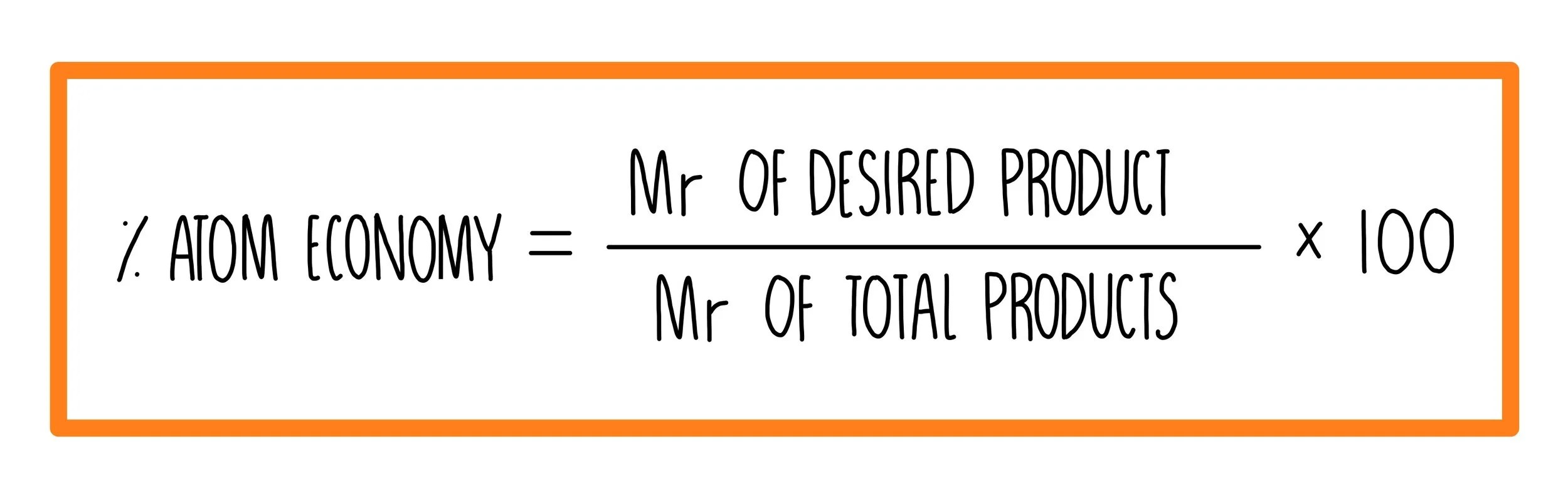
Of a reaction is a measure of the amount of starting materials that end up as useful products... Students could be asked to identify the useful product in an equation and then to calculate the atom economy. The greater the value of the %atom economy, the less the amount of waste product produced. Based on actual quantities of reagents used.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

The percentage atom economy of a reaction is calculated using this equation: Of a reaction is a measure of how many reactant atoms form a desired product. Of a reaction is a measure of the amount of starting materials that end up as useful products. Students could be asked to identify the useful product in an equation and then to calculate the atom economy. May 19, 2021 · atom economy is the molecular mass of the desired product ÷ the sum of molecular masses of all the products × 100. Based on actual quantities of reagents used. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product): The greater the value of the %atom economy, the less the amount of waste product produced. Jan 08, 2018 · to calculate the percent atom economy, we divide the atoms of desired product by the total atoms in the reactants (which is the same as the total atoms in the product):
